11/17/2003
tcaw-thermodynamics-1103.doc
1823 words
By Stephen R. Heller (Today’s
Chemist Not at Work)
11/17/2003
tcaw-thermodynamics-1103.doc
1823 words
By Stephen R. Heller
steve@hellers.com
If Even if you
passed Pchemphysical chemistry as an undergraduate,
you may well have struggled with obtaining data such as Gibbs free energy, entropy, and enthalpy for thermodynamic
equations., For years, those who
needed these vales had no choice but to look them up in one of the
numerous chemical data handbooks. Tyou probably
struggled with thermodynamics, and wondered if there was a better way. Well, today
there is a better way to obtain thermodynamic data; the Web. There are dozens
of sources of thermo data available on the web. The University of Illinois at Chicago (UIC) has an excellent web
page with links to over 100 Data & Property
Calculation Websites (3). This article
will cover just a few of the major sites, both free and paid, which are now
available. These include: the NIST
WebBook, DIPPR (4), Dechema (5), and FIZ-Berlin Infotherm (6)
According to NIST, usage of the web based WebBook (1)
database and search system exceeds 20,000 users per week with over 600,000
different IP addresses per year accessing just the WebBook. While the WebBook covers a wide variety of
subjects, its core is thermochemical and thermophysical data. The NIST WebBook
is not the only source of thermodynamic data and there are a number of other
thermodynamic databases, including the newest such system, the FIZ-Berlin
Infortherm system (2).
What are people doing with this data? The need and value of thermodynamics data is quite varied and deep. In the chemical process industry it would be difficult to build an efficient and cost effective plant for a chemical without good, high quality thermophysical and thermochemical properties. In the field of petroleum and bulk chemicals, where profit margins are slim, good data can be the difference between a well run profitable operation or one that is in the red. This data is needed for scale up testing of large batches of chemicals. Thermodynamics data is used to design systems to minimize heat losses. Thermo data helps in the design of distillation columns. In these time, when everyone is very cost sensitive, good thermo data will both improve the quality of the production as well as improve the quality, leading to better profit margins.
NIST Chemistry WebBook
The NIST Chemistry WebBook is by far the most well used
source of thermodynamics data. At
present all the information the WebBook is free, which in addition to its high
quality of evaluated data, is probably the major reason for its high and
widespread usage. It contains data on over 48,000 chemicals including thermochemical
data for over 7000 organic and small inorganic compounds such as
o
Enthalpy of formation
o
Enthalpy of combustion
o
Heat capacity
o
Entropy
o
Phase transition enthalpies and temperatures
o
Vapor pressure
and reaction thermochemistry data for over
8000 reactions on
o
Enthalpy of reaction
o
Free energy of reaction
The WebBook has numerous, easy to use, search capabilities including: chemical name, formula, molecular weight, CAS Registry number, author, structure, substructure, and reaction.
DIPPR - AIChE
DIPPR, the Design Institute for Physical Properties is a part of
The American Institute of Chemical Engineers, AIChE. DIPPR, with funding from the chemical industry, has undertaken
seven major projects for over the past two decades. The main project which is the subject of this article is Project
801 - Evaluated Process Design Data. This database of 1743 chemicals has become
the industry standard for pure component physical properties.
The DIPPR database consists of experimental data, estimated values
where necessary, temperature-dependent correlation coefficients, references,
notes, quality codes, and other information required for proper use of the
DIPPR database in computer-accessible form together with software for
searching, accessing, and using the data and accompanying information.
The database includes source data values and references, data
quality codes, and background information, such as molecular structure,
synonyms, hazard properties, miscellaneous properties, notes, and explanations.
It now contains data for 29 fixed-value properties and 15 temperature-dependent
properties. Data for approximately 1811 industrially important compounds are
included. As far as possible, values for all these properties for each chemical
are entered. If experimental data are not available, values are estimated when
possible. Temperature-dependent correlation coefficients, applicable upper and
lower temperature limits, and values computed at these limits are included for
temperature-dependent properties. Fundamental SI units are used. Some of the DIPPR data is available at no
cost. The data is very useful for
modeling programs. The database is available online via the Chemical Abstracts
Service STN computer network (7). The
database is also available in a limited form at Brigham Young University. If
you are a student or employee of an educational institution and would like
access to the DIPPR 801 student database, please go to the BYU website listed
in the references (8).
DECHEMA
The
DECHEMA (Society for Chemical Engineering and Biotechnology) is a non-profit
making scientific and technical society based in Frankfurt/Main, Germany. It
was founded in 1926. Nowadays it has over 5000 private and institutional
members. One of their main activities has been the creation and
dissemination of the Detherm database and search system.
The Detherm database provides
thermophysical property data for about 21,000 pure compounds and 101,000 mixtures.
These data are indispensable for the design of apparatus and chemical
production facilities. .
Detherm contains Literature
values, together with bibliographical information, descriptors and
abstracts. At the time 4.2 million data sets are stored
The following properties are stored:
·
phase
equilibrium data
·
vapor
pressures, critical data
·
thermodynamic
properties
·
transport
properties
·
surface
tensions
·
electrolyte
data
Infotherm - FIZ Berlin
The last of the database and search systems to be described
here is the Infotherm system from FIZ-Berlin -the "Chemistry Information
Centre" (Fachinformationszentrum Chemie, FIZ Chemie Berlin. Launched late
in 2003 this database of the thermophysical properties of substances
"Infotherm" in XML. XML (Extended Mark-up Language) is the standard
for information services in the Internet and in Internet-based company networks
(Intranets). By making Infotherm available via the Web, FIZ Chemie Berlin hopes
to target those chemical engineering offices and companies who are increasingly
using the Internet to meet their information requirements. The integration of
the database in company Intranets is simple using XML technology.
The quality Infotherm database delivers
data on pure compounds and on mixtures, such as PVT properties, phase
equilibria, transport and surface properties, calorific properties,
solid-liquid equilibria. The complete database currently contains more than
90,000 data records for mixtures and about 199,000 records for pure substances.
The first release in late 2003 contained more than 80,000 data sets on mixtures
and more than 60,000 on pure compounds. Each piece of data may be accessed via
the chemical name, the trivial name, molecular formula, or via the CAS Registry
Number. The data originates from journal articles, handbooks and data
collections which are evaluated by FIZ Chemie Berlin and which cover the
time-period from 1985 until the present. The database is being updated monthly.
The database provides information on the thermophysical properties of 6,300
pure chemical compounds and 23,000 mixtures along with full bibliographic data.
150,000 tables contain PVT-properties, phase equilibria, transport and surface
properties, caloric properties, and acoustic and optical properties. Searching
the database and the "preview" display format of results is free of
charge. This policy is somewhere
between the NIST data which is available at no cost, and the Dechema data which
is all for a fee. The DTherm does let you know when the information is
available at no cost. Infotherm offers pay-per-view access or full access to
all documents on a license basis. Pay-per-view users can purchase full access
to an individual document from the free preview format. For considerable
flexibility, all purchased documents can be downloaded in several data formats.
Note: The CSV
(comma separated value)-download exports only the data in a
spreadsheet format (for e.g.. MS-Excel). Optionally, all documents can be
stored in the “my infotherm” section by selecting from the hit list. These
documents will be available in future sessions. Besides licensing the entire
database customers can also purchase pre-paid packages whichpackages, which
can be transformed into an unlimited license if the usage exceeds the
respective license fee.
Data for the Antonine equation
An example of search in three of these systems and the
resulting output is shown belowin figures 1-3. In all the examples the search was for Antoine Equation data for
benzoic acid to
estimate the vapor pressure at different temperatures.
While cChemists often
use the Clausius-Clapeyron equation
to estimate the vapor pressures of pure liquids or solids and it works well for
most applications. Several of the assumptions made in the derivation of the
equation fail at high pressure and near the critical point, giving inaccurate
results. Chemical engineers often need more reliable vapor pressure estimates.
The Antoine equation is a simple 3-parameter fit to experimental vapor
pressures measured over a restricted temperature range:
|
log P = A - |
B |
where A, B, and C are "Antoine coefficients" that vary from substance to substance. Sublimations and vaporizations of the same substance have separate sets of Antoine coefficients, as do components in mixtures. The Antoine equation is accurate to a few percent for most volatile substances (with vapor pressures over 10 Torr).

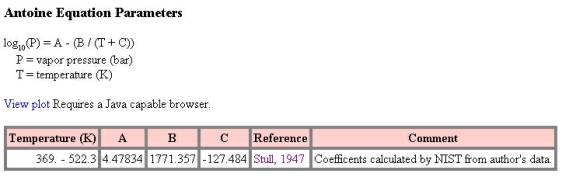
Figure 1 – NIST WebBook Antoine Equation Data for Benzoic Acid
The example below is the result
of a search for Antoine equation data on Benzoic Acid data. All data from the Dechema database is
available for a fee, the amount of which is shown in each of the 5 hits in
Figure 2.
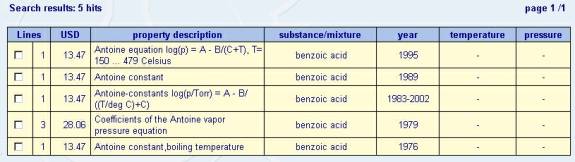
Figure 2 – DTherm Antoine Equation Data for Benzoic acid
The search below in the Infotherm system starts out with the web page displaying the search options, including search by name, formula, CAS registry number, the type of data you want to search for (the pull down menus list dozens of properties, similar to that found in the Dechema search system), a bibliography search for author, title, and so on, the type of system (pure, binary, and then up to denary) and the box at the bottom displays the search results.
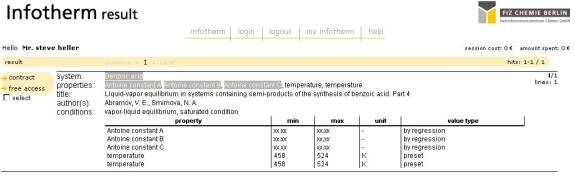
Figure 4 – Infotherm Antoine Equation Data summary output for Benzoic Acid
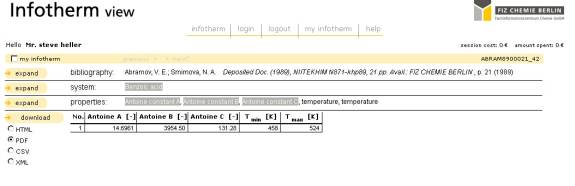
Figure 5 – Infotherm Antoine Equation Data complete output for Benzoic Acid
Figures 4 and 5 shows the hit resulting from the search and the detailed output. In this case, as the example chosen is one of those available at no cost, it is possible to show an entire Infotherm record.
In summary, thermodynamics data, a very important need for much industrial research, is now being made more widely available via the web. The examples shown here are just a few of the many sources of this information and again proving why the web is such a wonderful resource in making it easier for today’s chemist to do their work most quickly and efficiently.
References
2. http://www.fiz-chemie.de/infotherm/servlet/infothermSearch
3. http://tigger.uic.edu/~mansoori/Thermodynamic.Data.and.Property_html
4. http://www.aiche.org/dippr/
5. http://www.dechema.de/f-infsys-e.htm
6. http://www.fiz-chemie.de/infotherm/
8. http://dippr.byu.edu/students/chemsearch.asp