Fausto Ramirez, A. V. Patwardhan, and Stephen R. Heller
Department of Chemistry
State University of New York
Stony Brook, New York
Sir:
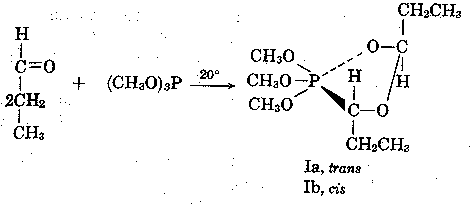
We wish to report the isolation and characterization of a tetraoxyalkyl
phosphorane (I) from the
reaction of 3 moles of anhydrous propionaldehyde
with 1 mole of trimethyl phosphite at 20o.
The 2:1 adduct I was obtained in ca. 60% yield based
on reacted phosphite (ca. 60%) after 14
days.

The 2:1 adduct I had a b.p. 50-51o (0.2 mm.) , n25 D 1.4313. Anal. Calcd for C9H21O5P; C, 45.0;
H. 8.7; P, 12.9; mol. wt., 240. Found: C, 44.9; H 8.8; P, 12.9; mol. wt. 275. There were strong
infrared bands at 9.10, 9.309, and 9.43 microns due to
CH3OP vibrations; no CO or PO
absorption; H1 n.m.r. (Neat, 60 Mc, τ): multiplets at
5.4 and 6.3 due to methine protons,
a doublet at 6.53 (JHP = 12.0 c.p.s) due to three methoxyls, multiplet at 8.4 (methylenes), and a
triplet at 9.06 (methyls).
Table 1
Chemical Shifts in the P31 N.M.R. Spectra of
Oxyphosphoranesa
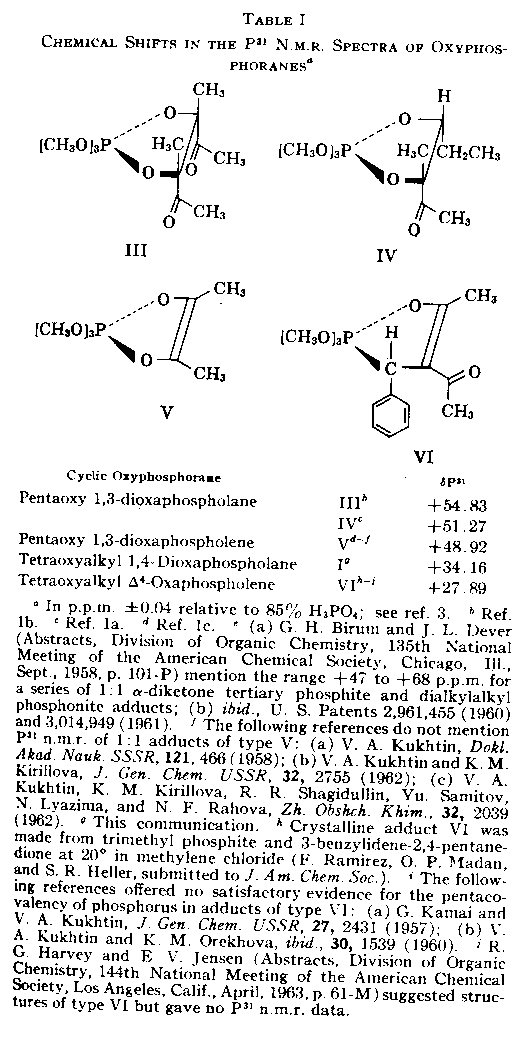
Figure 1.
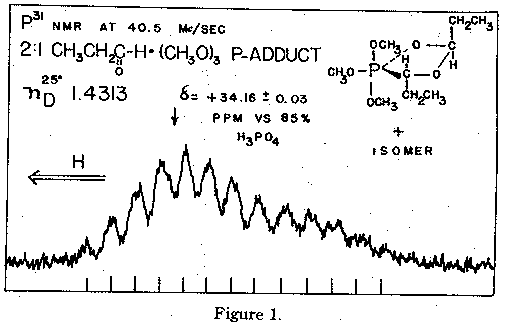
Further examination of the methoxy doublet at 100 Mc./sec disclosed the presence of two
doublets, both with JHP = 12.0 c.p.s. and separated by 0.5 c.p.s. This is attributed to the presence
of two diastereoisomers, Ia and Ib, for the following reasons. (1) The relative proporation of the
isomers could be altered somewhat by fractional distillation. (2) The P31 n.m.r. spectrum3 (Fig.
1) showed an unsymmetrical multiplet, suggesting the presence of two very similar phosphorus
nuclei. The multiplet due to the major isomer is centered at +34.16 +- 0.03 p.p.m. vs. 85%
H3PO4; that of the minor isomer at +32.8 p.p.m.. Data for other oxyphosphoranes4 are
summarized in Table I. The positive shifts of these compounds with pentacovalent phosphorus
should be noted.5 (3) The 2:1 adduct I underwent rapid hydrolysis to dimethyl 1-hydroxy-propylphosphonate (II) and propionaldehyde.
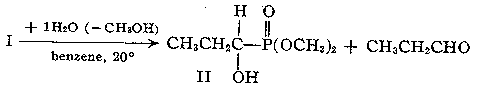
The hydroxy phosphonate II had b.p. 100-103o (0.5 mm), n25 D 1.4438. Anal. Calcd. for
C5H13O4P: C, 35.8; H, 7.8; P, 18.4. Found: C, 36.2; H, 7.9; P, 17.7. Bands were present at 3.08
(OH), 8.00, and 8.16 microns (PO). DELTA P31 = -31.1 p.pm.
The reaction of butyraldehyde with trimethyl phosphite was slower, but the course of the reaction
was analogous.
We conclude that the phosphorus of the phosphite adds slowly to the carbonyl carbon6 of the aliphatic aldehydes to form a 1:1 adduct VII, which reacts further with a second molecular of aldehyde to give the precursor VIII of the oxyphosphorane I. There is no alkyl group translocation at the 1:1 stage (VII) or at the 2:1 stage (VIII). However, we found evidence for a further reaction between VIII and the aldehyde to give a 3:1 adduct (similar to VIII) which underwent an alkyl group translocation to a 3:1 acetal phosphonate (IX).
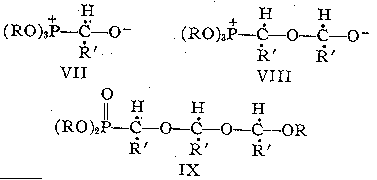
The reaction of triethyl phosphite with o-nitro-benzaldehyde,7 with benzaldehyde,8, and with
aromatic ketones9 has been reported. Expoxides and 1:1 adducts (analogous to VII) have been
isolated from the reaction of a phosphorus triamide with aldehydes.10
Acknowledgment. - We are grateful to Prof. P. C. Lauterbur of this department for advice on P31 n.m.r. spectroscopy and to Dr. E. M. Banas (American Oil Co.) And Prof. E. Eliel (University fo Notre Dame) for some earlier H n.m.r. spectra.
References
(1) (a) F. Ramirez, A. V. Patwardhan, N. B. Desai, R. Ramanathan, and C. V. Greco, J. Am. Chem. Soc., 85, 3056 (1963);
(b) F. Ramirez, R. Ramanathan, and N. B. Desai, ibid, 85, 3465 (1963);
(c) F. Ramirez, and N. B. Desai, ibid., 85, 3252 (1963); 82, 2652 (1960).
(2)Acknowledgment is made to the Cancer Institute of the National Institutes of Health (CY 04769) and to the National Science Foundatiuon (G 19509) for the support of this research.
(3) Varian HR 100 spectrometer at 40.5 Mc/sec
(4) In all the cases of Table I, except compound I, the P31 n.m.r. spectra were symmetrical, indicating the presence of one type of P-nucleus.
(5) See also Q. E. Thompson, , J. Am. Chem. Soc., 83, 845 (1961)
(6) Addition of the phosphorus of a phosphite to the carbonyl oxygen of a quinone was first observed by F. Ramirez and S. Dershowitz: (a) J. Org. Chem., 22, 856 (1957); (b)J. Am. Chem. Soc., 81, 4338 (1959); (c) see also ibid., 78, 5614 (1956).
(7) (a) V. A. Kukhtin and K. M. Kirollova, J. Gen. Chem. USSR, 31, 2078 (1961);
(b) Zh. Obshch. Khim., 31, 2226 (1961).
(8) A. Arbuzov and V. M. Zoroastrova, Izv. Akad. Nauk SSR Otd. Khim. Nauk, 1030 (1960).
(9) A. C. Poshkus and J. E. Herweh, Abstracts, Division of Organic Chemistry, 141st National
Meeting of the American Chemical Society, Washington, D.C., March 1962,
p. 17-O.
(10) V. Mark., J. Am. Chem. Soc., 85, 1884 (1963).
Received September 26, 1963